Biomarker
Biomarker
Biomarker is a indicator that can detect changes in biological state or condition of the body using cells, blood vessels, proteins, or DNA, etc. The first use of the word biomarker is the National Institutes of Health (NIH), which defines biomarkers as "a defined characteristic that is used as an indicator of normal biological processes, pathogenic processes or responses to a therapeutic intervention" . JW is currently accelerating the development of the kit by securing unique technologies for sepsis diagnostic biomarkersand pancreatic cancer diagnostic biomarkers.
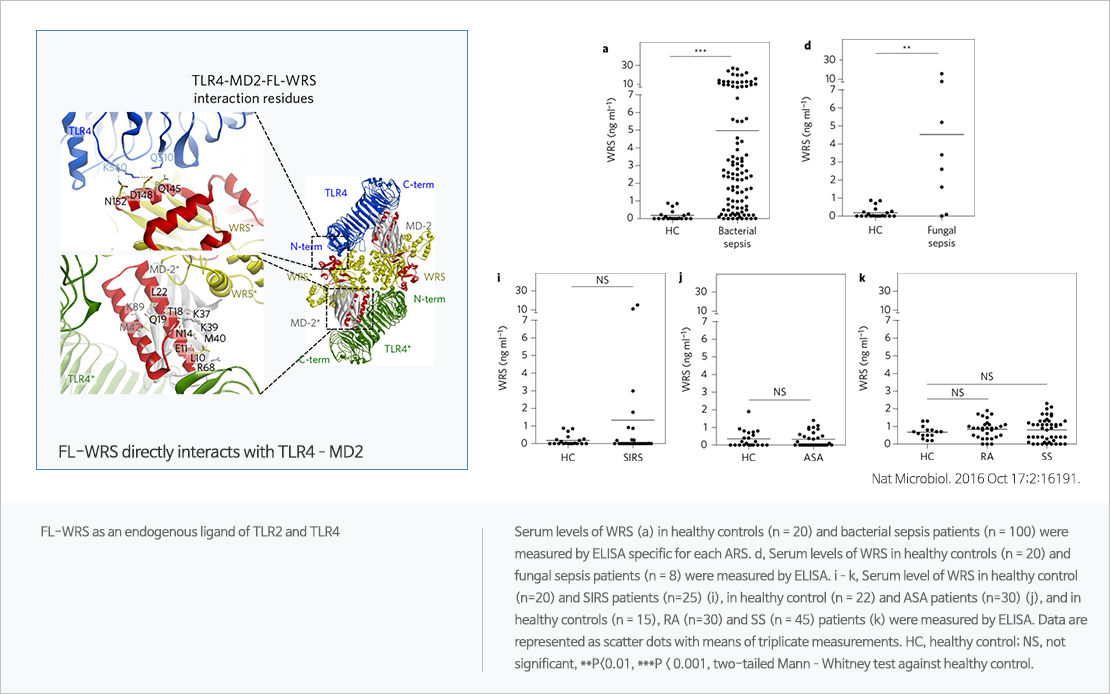
WRS, promising biomarker for sepsis
Sepsis refers to organ failure caused by disorders of the body's immune response regulation caused by infection, and is one of the high mortality diseases with 30 million cases worldwide each year. It is the most common disease in the intensive care unit in Korea. Currently, rapid response and continuous monitoring through early diagnosis are the only way to reduce sepsis mortality. WRS is a type of aminoacyl tRNA synthetase (ARS) that is primarily responsible for the synthesis of proteins in the body. In 2016, the world's first new cell regulatory network was identified at Nature microbiology, demonstrating its potential as a sepsis diagnostic biomarker. In this paper, we found that WRS is rapidly expressed in sepsis patients and can be diagnosed as sepsis due to various infections (bacteria, fungi, viruses). In-house clinical studies have shown that WRS reflects sepsis severity as well as high diagnostic accuracy.
Intellectual property
- Method of detecting a composition and diagnostic marker for diagnosing an infectious disease or complication using tryptophanyl tRNA synthase (2016)
Registered in K (10-1771697)
Patent application is filed (US/EP/JP/CN) and under examination.
- Antibodies that specifically bind to WRS proteins and uses thereof (2019)
(10-2019-0087230, 10-2019-0087233)
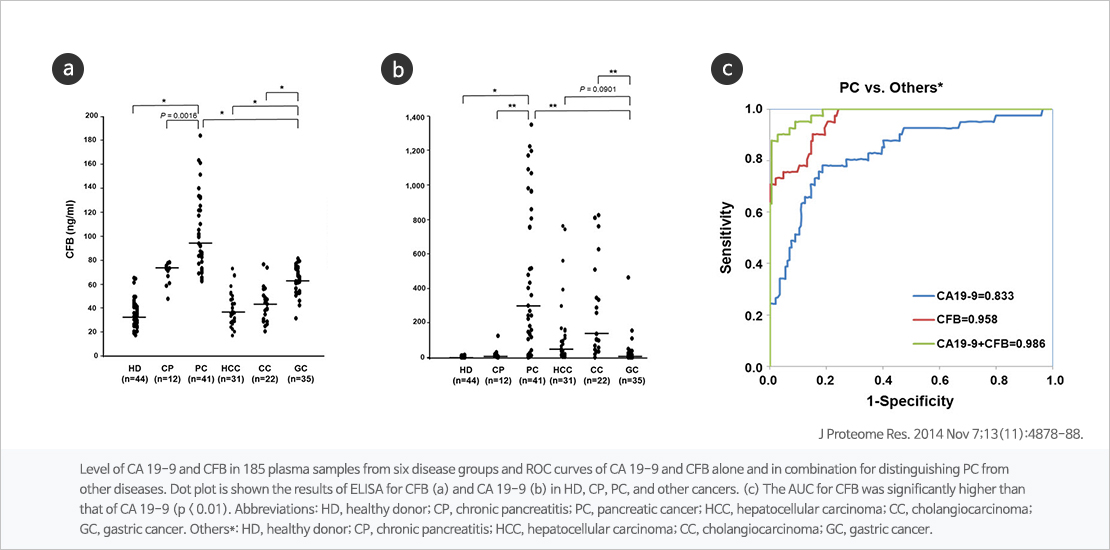
Combination of CFB and CA19-9, novel biomarker for pancreatic cancer
Pancreatic cancer is the fourth highest death rate in the world and fifth in the country, with a five-year survival rate of only 10 percent. Pancreatic cancer has no initial symptoms and is difficult to detect, and is mostly diagnosed at the end of the year, except for early detection, has a poor prognosis and a high mortality rate. Early diagnosis is essential to reduce the mortality of pancreatic cancer, but carbohydrate antigen 19-9 (CA19-9) currently used as a pancreatic cancer diagnostic biomarker is less accurate for early diagnosis.
The technology for multi-viomarker analysis using the new pancreatic cancer biomarker, B (CFB) and CA19-9, has been found to improve the accuracy of the existing pancreatic cancer diagnosis biomarker, CA19-9. In addition, early diagnosis of pancreatic cancer has been proven possible.
 Diagnostic Values of CA 19-9 and CFB According to Cut-off Levels in PC versus Other Disease
Diagnostic Values of CA 19-9 and CFB According to Cut-off Levels in PC versus Other Disease
|
CFB (ng/mL) |
CA 19-9 (U/mL) |
CFB1 + CA 19-9 |
| PC vs Others |
78.42 |
37 |
- |
| Y-index |
71 |
50.4 |
87.4 |
Sensitivity
(%; 95% CI) |
73.1
(72.4-73.7) |
80.4
(79.8-81.0) |
90.1
(89.7-90.6) |
Specificity
(%; 95% CI) |
97.9
(97.8-98.1) |
70.0
(69.6-70.4) |
97.2
(97.0-97.3) |
1Sensitivity and specificity of the combination of CFB and CA 19-9 from the maximum Youden’s index.
2Predicted optimal cut-off value from the maximum Youden’s index.
Abbreviations: CA19-9: carbohydrate antigen 19-9; CFB: complement factor b; PC: pancreatic cancer; Others: HD (healthy donor), CP (chronic pancreatitis), HCC (hepatocellular carcinoma), CC (cholangiocarcinoma), GC (gastric cancer)
Publication
Identification of Human Complement Factor B as a Novel Biomarker Candidate for Pancreatic Ductal Adenocarcinoma J Proteome Res. 2014 Nov 7;13(11):4878-88
Intellectual property
- Pancreatic cancer diagnostic kit including antibody that specifically binds to complement factor B protein (Registration No. 10-1594287)
- Patent registered in KR/EP/JP/CN/US
POCT
POCT (Point of Care test)
JW Bioscience is developing an easy to use, bench-top chemiluminescent immunoassay analyzer providing high-quality results from blood samples within 20 minutes.
- Compact immunoanalyzer with full automation
- High level of accuracy from combination of chemiluminescence technology
- Single test reagent cartridge
- Six simultaneous tests in under 20 minutes
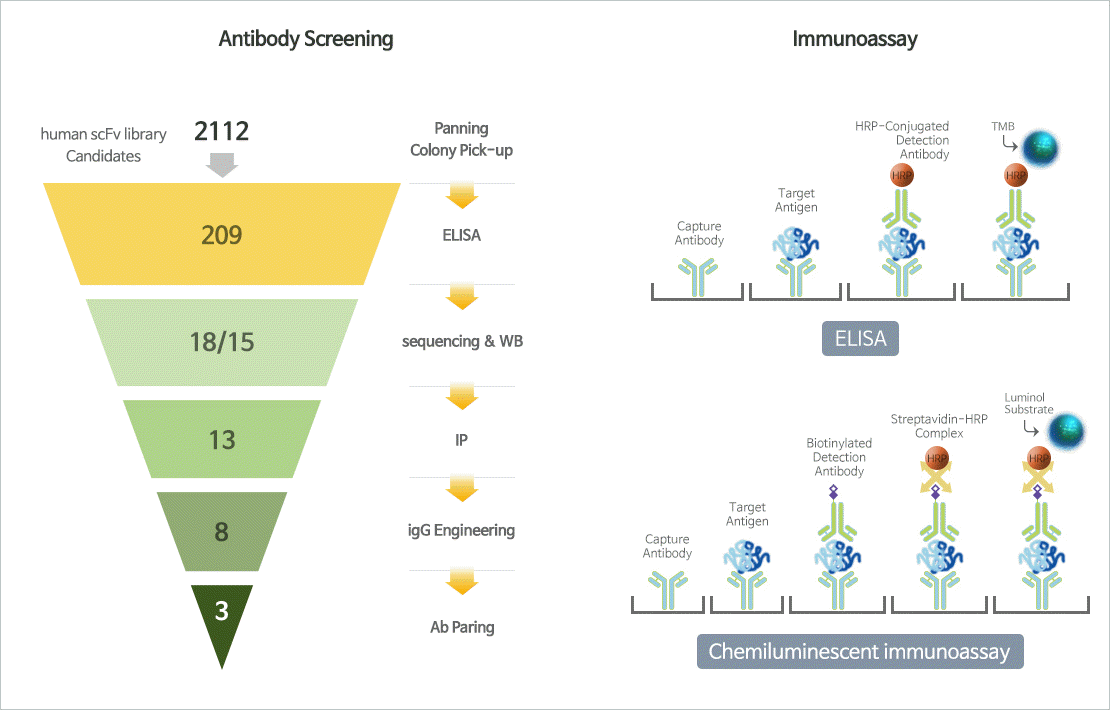
Immunoassay
Immunoassay
- Immunoassay is an biochemical test that measures the target analyte (ex. Protein) using antigen-antibody responses. Immunoassay can be used to diagnose diseases such as cancer, infectious diseas and immune disorders, and so on.
- JW has antibody screening technology for select, antibody candidates for biomarkers, and optimizing immunoassay methods using a variety of buffers and substrates.

 Diagnostic Values of CA 19-9 and CFB According to Cut-off Levels in PC versus Other Disease
Diagnostic Values of CA 19-9 and CFB According to Cut-off Levels in PC versus Other Disease