Pipeline
X
 Sepsis is the most common disease in the intensive care unit, and mortality rates from 40% to 70% if not detected early and treated. Although early diagnosis and early treatment are important prognostic factors, no effective screening methods have been developed.
Sepsis is the most common disease in the intensive care unit, and mortality rates from 40% to 70% if not detected early and treated. Although early diagnosis and early treatment are important prognostic factors, no effective screening methods have been developed.
JW strives to develop diagnostic reagent enabling quick and accurate diagnosis before progress to sepsis.
Current goal is to develop reliable diagnostic kit capable of early diagnosis and mortality prediction of sepsis.
Sepsis
 Sepsis is the most common disease in the intensive care unit, and mortality rates from 40% to 70% if not detected early and treated. Although early diagnosis and early treatment are important prognostic factors, no effective screening methods have been developed.
Sepsis is the most common disease in the intensive care unit, and mortality rates from 40% to 70% if not detected early and treated. Although early diagnosis and early treatment are important prognostic factors, no effective screening methods have been developed.JW strives to develop diagnostic reagent enabling quick and accurate diagnosis before progress to sepsis.
Current goal is to develop reliable diagnostic kit capable of early diagnosis and mortality prediction of sepsis.
X
 Since pancreatic cancer is asymptomatic and effective screening tests have not yet been developed, it is often diagnosed after the disease progressed
Since pancreatic cancer is asymptomatic and effective screening tests have not yet been developed, it is often diagnosed after the disease progressed
At the time of diagnosis, 40 to 50 percent of the metastases were already found.
Pancreatic cancer is a disease with high medical unmet needs with a survival rate of 5% or less within 5 years.
JW's goal for pancreatic cancer diagnostic kit is to examine pancreatic cancer by stages of progression using biomarkers expressed at the early and late stages of pancreatic cancer and to predict recurrence after treatment.
JW is developing multi-biomarker measurement kits and diagnostic algorithms based on novel biomarkers having high sensitivity and specificity for pancreatic cancer that offer higher accuracy than existing diagnostic tests.
Pancreatic cancer
 Since pancreatic cancer is asymptomatic and effective screening tests have not yet been developed, it is often diagnosed after the disease progressed
Since pancreatic cancer is asymptomatic and effective screening tests have not yet been developed, it is often diagnosed after the disease progressed At the time of diagnosis, 40 to 50 percent of the metastases were already found.
Pancreatic cancer is a disease with high medical unmet needs with a survival rate of 5% or less within 5 years.
JW's goal for pancreatic cancer diagnostic kit is to examine pancreatic cancer by stages of progression using biomarkers expressed at the early and late stages of pancreatic cancer and to predict recurrence after treatment.
JW is developing multi-biomarker measurement kits and diagnostic algorithms based on novel biomarkers having high sensitivity and specificity for pancreatic cancer that offer higher accuracy than existing diagnostic tests.
JWBS-R001
Novel biomarker for sepsis diagnosis
WRS (Tryptophanyl tRNA synthetase)
- Novel biomarker for sepsis diagnosis
- WRS is involved in innate immune response and is rapidly released into the blood during infection
- Secreted level of WRS was increased in patients with sepsis caused by various types of infection (bacteria, fungi, viruses). WRS as a potential biomarker for sepsis diagnosis has been identified.
- Secreted tryptophanyl-tRNA synthetase as a primary defense system against infection (Ahn YH et al, Nature microbiology, 2016)
POCT (Point of care testing)
- Rapid diagnostic platform for measuring biomarkers in blood
- Automated analyzer based on CLEIA.
- Up to 6 tests per run in less than 20 minutes
- will be released in 2024
JWBS-R002
Novel biomarker for pancreatic cancer diagnosis
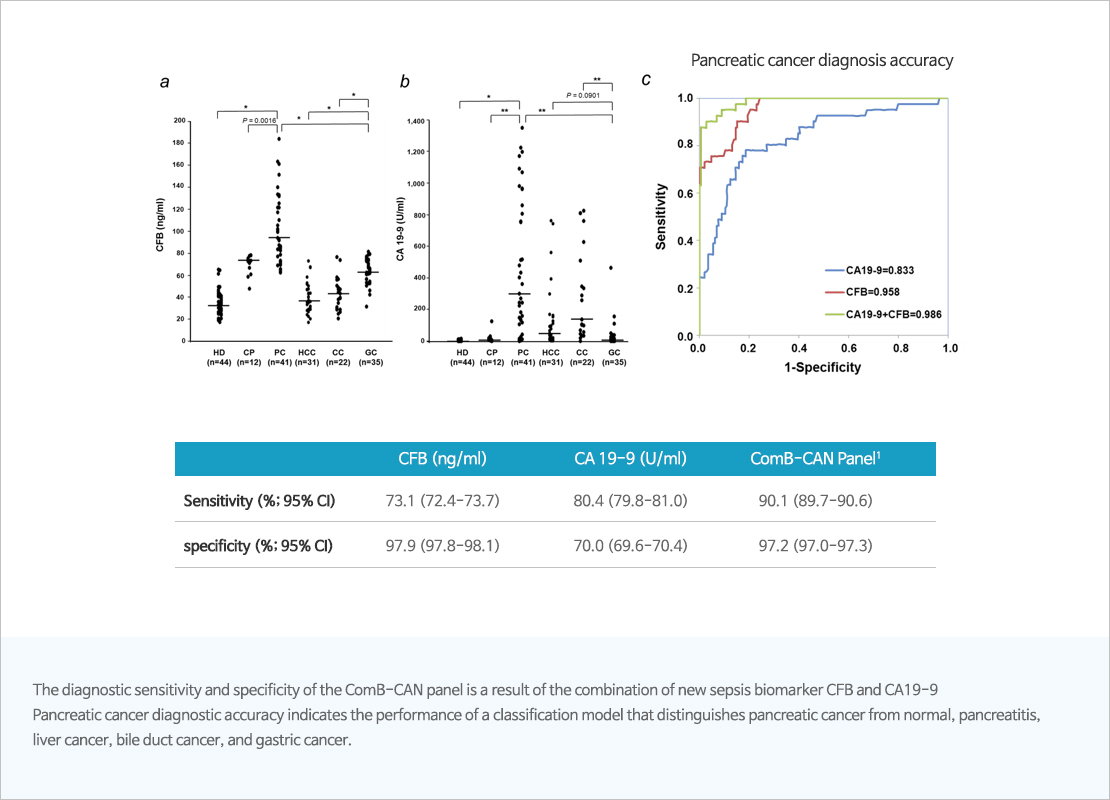
CFB (Complement factor B)
- Novel biomarker for early diagnosis of pancreatic cancer
- CFB is involved in the development and metastasis of pancreatic cancer and can be detected in the blood from the early stage of cancer.
- When combined with the existing pancreatic cancer biomarker CA19-9, CFB improves diagnostic accuracy of pancreatic cancer.
- Underdeveloping CFB and CA19-9 multi-biomarker measurement kits and analysis algorithms.
- Identification of Human Complement Factor B as a Novel Biomarker Candidate for Pancreatic Ductal Adenocarcinoma J Proteome Res. 2014 Nov 7;13(11):4878-88
- Early Diagnostic Ability of Human Complement Factor B in Pancreatic Cancer Is Partly Linked to Its Potential Tumor-Promoting Role. J Proteome Res. 2021;20(12):5315-5328.
POCT (Point of care testing)
- Original technology patent registration (10-1594287)
- Patent application status
-
R&D
Information - Research direction
- Technology
- Pipeline
-
Open
innovation